What benefits will COVID-19 tests have for my business?
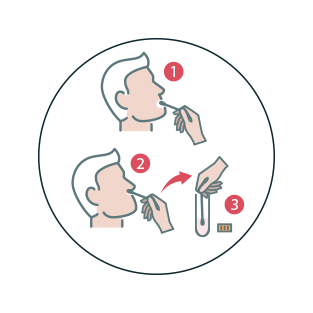
PCR Swab Test
- Offer a Fit-to-Fly service for your patients, most airlines and countries do not accept the NHS PCR Swab tests for proof of a negative COVID-19 result!
- Can be carried out in the clinic by a healthcare professional or provided to your patients to carry out at home.
- Allows you to test following an infection to detect if the virus has been cleared.
- Screen asymptomatic individuals to detect if they are carrying the virus and are potentially infectious to others who are at high risk in the workplace and elsewhere.
- Use in combination with Antibody Testing as a dual testing strategy for optimum results.
Rapid Antigen Test
- Detects an active Coronavirus infection in just 10 minutes.
- Cost effective and time efficient with results in just 10 minutes anytime and anywhere.
- Ideal for testing patients upon arrival to the clinic to provide peace of mind for yourself and your colleagues.
- Waiting for your PCR test to arrive but want that peace of mind instantly? This test does exactly that.
- Individuals who want to book a holiday but concerned they will get a positive PCR test although they are asymptomatic, this test is 99.3% accurate.
- Efficient and easy on-site testing for your colleagues to support a return-to-work strategy.
Rapid Antibody Test
- On-site testing for your colleagues and your patients, with results in approximately 10 minutes.
- Knowing if you, your colleagues or your patients have developed the Antibodies against the virus.
- This test can be offered to patients who have surpassed the required time frame following symptoms required for optimum PCR test results, where PCR testing alone may not provide an accurate diagnosis.
- If you think you had Coronavirus but testing wasn’t readily available at the time, this test will provide a good indication.
Did you know...
Combining PCR an Antibody Testing can greatly increase test result reliability?
The science speaks for itself, a recent study by the University of Cambridge showed that combining both PCR and Antibody testing was superior to virus detection alone and can significantly improve the diagnosis of COVID-19.
“Combining point-of-care PCR and Antibody testing could be a game-changer for rapidly identifying those patients with moderate to severe COVID-19 infection”
Professor Ravi Gupta, Institute of Therapeutic Immunology and Infectious Disease, University of Cambridge. Full report available here
It can take up to 14 days for an individual to start showing COVID-19 symptoms, by which time the virus may have moved from the nose and throat into the lungs. Antibodies on the other hand, particularly IgM antibodies can be produced much sooner so a combination of both tests, can greatly increase the accuracy of diagnosis.
Results of the study found that “the nucleic acid tests could identify eight out of ten patients with COVID-19, but when combined with the rapid antibody tests, 100% of the COVID-19 patients were correctly identified”
What’s the difference between the COVID-19 PCR Swab Test & the Rapid Antibody Test?
The PCR Swab test detects viral RNA and therefore an active infection, whereas the Rapid Antibody Test detects the antibodies your body produces following an infection.
Think you might have had Coronavirus in the recent past but not sure because testing methods weren’t readily available? Check if you have developed the antibodies against the virus with our Rapid Antibody Finger-prick tests here
How do I decide what test is best for myself, my patients, my colleagues and my clinic?
In conclusion…
The best in class detection method for an early infection is a dual approach of PCR and Antibody testing, here’s why:
- A dual testing approach of using low-cost Antibody testing combined with PCR testing to confirm a positive IgM Antibody result during the early stages of an infection (days 1-7 following symptoms) will enable organisation to deploy a safe return-to-work strategy.
- Offering both Antibody and PCR testing in your clinic will enable you to provide a range of services catering to varying patient needs.
- Antibody testing can be offered as a method to support diagnosis of acute COVID-19 illness for patients who present late i.e. 9–14 days after symptoms onset. During this time period, the sensitivity of RNA detection is decreasing, and the sensitivity of antibody testing is increasing so combine both to increase the accuracy of the diagnosis!
Why not consider COVID-19 testing opportunities for your clinic?
Terms and conditions:
*The lab endeavour to provide your results 24 hours from receipt of sample, however in the case of inevitable postage delays and repeat sample analysis, results can be delayed.